

The N shell containing 4s, 4d, 4p and 4f, can carry 32 electrons. The M shell contains 3s, 3p, and 3d, and can carry 18 electrons. The K shell contains a 1s subshell hence it can carry 2 electrons, the L shell has 2s and 2p, and can carry 8 electrons. This decides the electron capacity of the shells. The maximum electrons that can be carried by the sub-shell S is 2, by P is 6, by D is 10, and the F sub-shell can carry 14. Each shell and subshell have a limitation on the amount of electrons that it can carry. lab Hydrogen quantum numbers atomic orbitals and electron configurations. The subshells have a distinct shape and configuration, in which the electrons move freely. that lanthanum La is the first element in the 4f block and that actinium Ac. A quantum number beginning in n 3, 0, describes an electron in the s orbital of the third electron shell of an atom. They stand for sharp (S), principal (P), diffuse (D), and fundamental (F). The shells are labeled K, L, M, N, and so on, from the innermost to the outermost shell.Įach shell has subshells that are named for the type of emission lines produced from different states of angular momentum. Now for the angular momentum quantum number, l, which describes the subshell in which the electron resides.
So, the highest-energy electron found in gallium is located in a 4p-orbital, which means that right from the start you know that the value of its principal quantum number, n, will be 4. Each has its own specific energy level and properties.

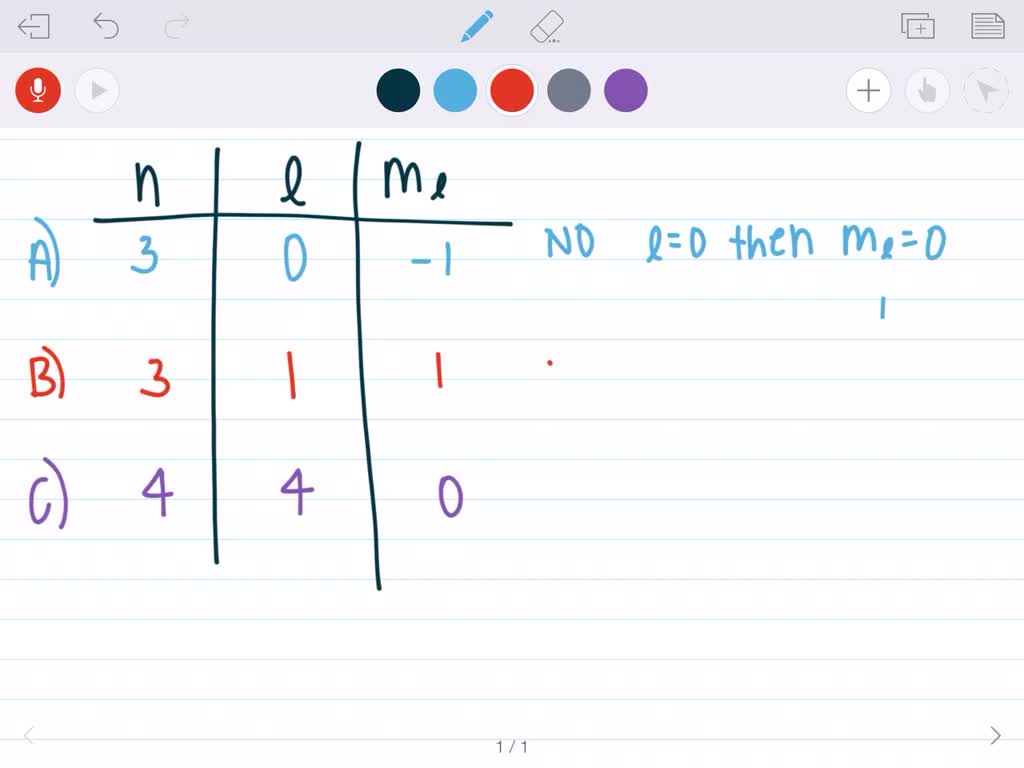
This model has been widely accepted, and according to it, each atom has shells, which further have subshells. As you know, the quantum numbers are defined. Quantum Numbers describing Electronic Orbitals There are multiple orbitals within an atom. The angular quantum number (l) can be any integer between 0 and n- 1. The allowed values of nare therefore 1, 2, 3, 4, and so on. The principal quantum number (n) cannot be zero. What are the allowed values for the me quantum. A Radial Node can be defined as the spherical area near the elements nucleus where there is a. The three quantum numbers (n, l, and m) that describe an orbital are integers: 0, 1, 2, 3, and so on. What are the allowed values for the n and l quantum numbers for a 4d orbital b. It involves the specific arrangement of electrons in shells and sub-shells of Bohr’s atomic model. The total number of angular nodes found in the 4d orbital is two. The concept of electronic configuration has replaced the older concept of valency and valence electrons. The electronic configuration of each element is decided by the Aufbau principle which states that the electrons fill orbitals in order of increasing energy levels.


 0 kommentar(er)
0 kommentar(er)
